OrthoIndy cartilage restoration specialist, Dr. Jack Farr is now accepting patients for a new knee study. The KineSpring trial predated the current Atlas™ knee study. The KineSpring trial is fully enrolled and now patients are undergoing serial follow-up on their outcomes. The Kinespring was an implant along the inner (medial) aspect of the knee for mild to moderate medial knee arthritis. This study evaluated the device’s ability to decrease the arthritic pain. It was largely effective in decreasing medial knee arthritic pain, yet was slightly bulky in some patients.
Study details
The concept of unloading the medial knee to treat medial arthritis is not new. In the 1950s and 1960s, cutting the tibia and realigning it from a bowed knee to a straight or mildly knock-knee alignment decreased the medial knee forces and decreased medial arthritis pain. High tibial osteotomy (video) remains effective today and is offered at OrthoIndy when indicated.
Alternatively, a partial knee (also offered at OrthoIndy) can resurface the involved medial side of the knee. While partial knee replacements are quite effective in relieving knee pain when there is end-stage, single compartment arthritis, it involves bone cuts and implantation of metal and plastic. Some patients with symptomatic, but less than end-stage arthritis are looking for an alternative that does not cut or alter the bone structure. They may try an unloader brace (available through OrthoIndy with prescription), yet often tire of reapplication daily. This patient with symptomatic mild to moderate medial arthritis is the focus of the KineSpring and now Atlas devices; they decrease medial knee loading without cutting or realigning the bones AND do not preclude future realignment, partial or full knee replacement.
Study enrollment
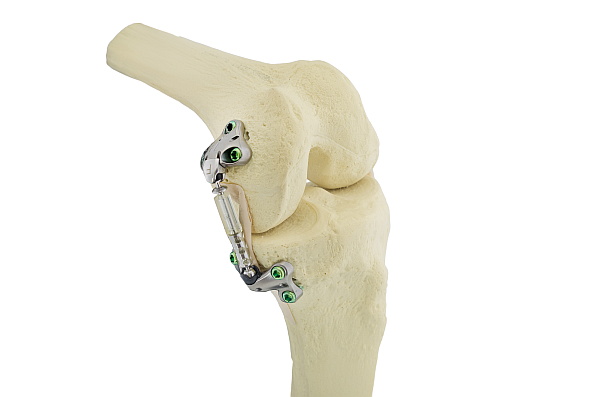
The Atlas is the “newest model” of the medial unloader family. It is a shock absorber rather than a spring and has a much lower profile allowing a smaller incision than the KineSpring. Early patient outcomes in Europe are encouraging. The FDA has approved a US trial. OrthoIndy is a participating site for the study. Additional information is available at www.atlaskneestudy.com.
For those patients who do not want any type of surgery, in addition to weight loss, physical therapy and oral medications, there are a variety of knee injections to consider—including “biologics.” OrthoIndy often participates in clinical trials of biologic injections. For those interested, please visit Dr. Jack Farr’s Research page.
To make an appointment with Dr. Farr please call 317.884.5163 or learn more about cartilage restoration.
Schedule an appointment
Your well-being is important to us. Click the button below or call us to schedule an appointment with one of our orthopedic specialists. If your injury or condition is recent, you can walk right into one of our OrthoIndy Urgent Care locations for immediate care. For rehabilitation and physical therapy, no referral is needed to see one of our physical therapists.